Nov 06
Help Us Protect Access to Sexual and Reproductive Health Care Today!
 September 28th is the 20th birthday of mifepristone, the pill commonly used for medication abortion and early pregnancy loss care!
September 28th is the 20th birthday of mifepristone, the pill commonly used for medication abortion and early pregnancy loss care!
For 20 years, mifepristone has helped people in the United States achieve their rights to bodily autonomy and to decide if and when to have children. And, more recently, it has supported pregnant people to manage their miscarriages in safe, effective ways that align with their preferences for care. Despite these achievements, in the U.S. people still do not have true, equitable access to mifepristone. Persistent barriers, like FDA requirements to stock and dispense the pill in-clinic, prevent pregnant people from accessing the timely, compassionate care they need. Many state laws prohibit telemedicine for abortion, prevent advanced practice clinicians from providing, and mandate counseling with false information. This inequitable landscape continues to disproportionately impact Black, Indigenous, and People of Color, and rural and low-income populations.
Join us this month on social media (Facebook, Instagram, Twitter) in celebrating mifepristone and recognizing the gaps to access and equity that persist. Every day through September 28th we’ll share clinician stories, resources, and actions you can take to learn more and spread the word about the need to make mifepristone more accessible to all.
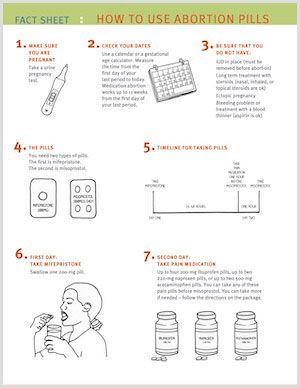 Using medication for an abortion is safe, effective, and the preferred option for abortion care by many. During the COVID-19 pandemic, medication has offered an opportunity to end a pregnancy in the safety of one’s own home rather than risking entering a clinic for a procedure. However, this medication is not new. Mifepristone combined with misoprostol has been used to end pregnancies in the U.S. for the past 20 years. And yet, many people do not know how a medication abortion works or what to expect when using medication for an abortion. This is why we created our newest resource: How to Use Abortion Pills fact sheet.
Using medication for an abortion is safe, effective, and the preferred option for abortion care by many. During the COVID-19 pandemic, medication has offered an opportunity to end a pregnancy in the safety of one’s own home rather than risking entering a clinic for a procedure. However, this medication is not new. Mifepristone combined with misoprostol has been used to end pregnancies in the U.S. for the past 20 years. And yet, many people do not know how a medication abortion works or what to expect when using medication for an abortion. This is why we created our newest resource: How to Use Abortion Pills fact sheet.
This new fact sheet explains each step that happens when using mifepristone and misoprostol for abortion care. Not only is it broken down into step-by-step instructions, but it also has images to help explain what happens during each step. Additionally, it includes information on what to expect, if and when you should reach out to a clinician for help, and when you can start using birth control.
Using mifepristone and misoprostol to end a pregnancy is a great option for people who might want not want to go through the abortion care process in a clinic – during COVID or otherwise – and it is very safe and effective. Patients should be provided with this option along with all the information they need to support them through this process. We encourage you to share the new How to Use Abortion Pills fact sheet, along with the rest of our resources!
We’ve been thinking about the virtues of going virtual long before the pandemic. In particular, how using virtual technology could help us expand our training and technical assistance programs nationally in creative and accessible ways. While we are able to teach the clinical management of medication abortion easily to large groups of clinicians (we have trained hundreds of clinicians all across the country), we have found that working through implementation issues takes a lot longer. Organizations need technical assistance, legal, and financial support to even be able to dispense mifepristone on site. Up until now, we have provided health care organizations with this support on a one-on-one basis. This fall we are laying the groundwork to launch a pilot “virtual knowledge network” in 2021 to scale up our technical assistance capacity.
Our virtual knowledge network pilot, or VKN, will allow us to connect with 15 – 20 health care organizations simultaneously, providing them with intensive technical assistance. These organizations will connect with us directly, but, even more powerfully, they’ll connect with each other. Interdisciplinary teams from participating organizations will attend hourlong structured online sessions every two weeks. We’ll cover core issues we have found that most organizations need to address to provide medication abortion. Each meeting will also have time to explore, in-depth, the experience of one of the participants. Organizations will learn from experts and from each other. We will study our process to figure out what the critical elements of the VKN should be. We’ll also create an online community where participants can communicate with each other and share resources, further reinforcing what they learn at each session.
This month, as we celebrate 20 years of mifepristone access in the United States, we are launching a fundraising campaign to raise $20,000 to help us launch of VKN pilot. A generous donor has pledged to match all donations, dollar for dollar, to up $10,000. Donate today to help us kick off this campaign!

On September 1st, our friend and colleague, Dr. Meera Shah, released her new book. You’re The Only One I’ve Told: The Stories Behind Abortion illustrates the complexities of abortion care through personal accounts, medical expertise, and an intersectional analysis of the abortion rights landscape in the United States.
You’re The Only One I’ve Told: The Stories Behind Abortion is available everywhere, but if you purchase here and use the code RHAP30 you get for 30% off the book. Proceeds from your order will be generously donated to the Reproductive Health Access Project!

As we celebrate the 20th anniversary of mifepristone, we want to look back on the history of this medication and how it has expanded the options available for those seeking abortion care.
French researchers developed mifepristone (also known as RU-486) in the 1980s, to be taken along with misoprostol for medication abortion care. Despite the outcry of anti-abortion activists, it was approved for use in France in 1988. In the United States, however, it was a very different story, as the FDA banned the importation of mifepristone in 1989. The manufacturer of mifepristone, Roussel-Uclaf, also banned mifepristone distribution in the United States and refused to supply it to researchers for investigating the drug’s other possible health benefits.
The first challenge of this ban in the courts was by an American woman named Leona Benten, who was stopped by U.S. Customs when bringing mifepristone into the country from Great Britain. Although Benten lost her case, it brought mifepristone into the national spotlight and galvanized the movement to overturn the FDA ban. When President Bill Clinton was elected in 1993, he ordered the Department of Health and Human Services to investigate mifepristone’s use for medication abortion. In 1995, Roussel-Uclaf agreed to give the Population Council the patent rights to mifepristone in the United States.
It wasn’t all downhill from there, however – although the FDA’s advisory branch recommended mifepristone for approval, legal and manufacturing troubles led to the medication being pushed to the side until 2000. Thanks to the work of activists and health professionals, mifepristone was finally approved for medication abortion care use on September 28, 2000, twelve years after its original synthesis.
Today, medication abortion care is provided up to 10 or 11 weeks’ gestation in the United States. According to the Guttmacher Institute, medication abortion accounted for 39% of all abortion care in the U.S. in 2017, up from 20% in 2014. Since 2001, medication abortions have increased from 5% of all abortion care to 30% in 2017. The percentage of abortions that are medication abortions continue to increase today, with many people opting to take the pills in the comfort of their own homes.
Unfortunately, mifepristone remains inaccessible to many people. The US Food and Drug Administration (FDA) regulates mifepristone under a Risk Evaluation and Mitigation Strategy (REMS), which means it’s classified as a “dangerous drug” – despite its history of safe and effective use. This classification prevents clinicians from prescribing mifepristone, but rather requires them to stock and dispense the pill directly to patients in-office. When mifepristone is inaccessible in primary care, patients are harmed. Their continuity of care is disrupted, emotional and financial consequences are exacerbated, they experience unnecessary and invasive procedures, and they must manage multiple appointments and delays in care.
With today’s ongoing global COVID-19 pandemic, this REMS rule requires patients to travel to the clinic to pick-up mifepristone in-person, forcing those seeking abortion care to risk needless exposure to the virus. In fact, the FDA has allowed patients to obtain nearly all other REMS classified drugs by mail to avoid COVID-19 risks, but not mifepristone. It must be picked up at a clinic though it can be taken later at home. In the spring, the American Civil Liberties Union (ACLU) filed a lawsuit on behalf of a coalition of medical experts and reproductive justice advocates to fight this rule. On July 13, 2020, a federal district court issued a preliminary injunction that blocks the FDA from enforcing its in-person pick-up requirement for medication abortion until at least 30 days after the end of the federal government’s declared public health emergency. This ruling is particularly important for low-income communities and those who are Black, Indigenous, and People of Color, as they make up the majority of those disproportionately impacted from COVID-19.
The preliminary injunction now allows clinicians who stock mifepristone to mail the pill to their patients for medication abortion. However, barriers still remain. For states that already outlaw telemedicine for medication abortion, this injunction does not apply. It also does not apply to patients seeking early pregnancy loss care. And, the Trump administration continues to challenge the July ruling. On September 8, the ACLU went to the Supreme Court to continue fighting the Trump Administration and to ensure that mifepristone can remain more safely accessible during the COVID-19 pandemic.

Over the next 20 days, check in with RHAP’s social media (Facebook, Instagram, Twitter) for stories and quotes from clinicians across the U.S. about mifepristone and why it’s a critical part of medication abortion and early pregnancy loss care. Here is an account from a family physician in Colorado about why they think it’s important to provide mifepristone in primary care:
“Mifepristone is the natural next step to comprehensive contraception care – in so many of the common but nuanced scenarios around preventing pregnancy, having options to respond when pregnancy does occur feels like we are doing our due diligence. We are now dispensing emergency contraception and – for patients who can afford the self-pay rates required by our health system – providing medication abortion.
We continue to be hamstrung by payor issues – Colorado Medicaid does not cover abortion, and we cannot provide free or discounted abortion care to Medicaid patients. We are pursuing the [steps] required to develop a self-pay package, but for now, there is a narrow slice of people who can afford the several hundred dollars required for the visits, testing, and med itself. But it has worked! Our clinic leadership has been wonderful and our nursing staff has been engaged and collaborative.”
If you are a clinician and have a story about mifepristone to share, please e-mail Marisa Peters, RHAP’s Social Media Consultant, at marisa@reproductiveaccess.org.

We are now accepting applications for fellows to start in the summer of 2021! This is a one-year, clinical fellowship open to board certified family physicians with the goal of developing leaders who will provide, teach and advocate for full-spectrum reproductive health care, including abortion, within primary care. We are recruiting for fellowship positions in Massachusetts, Michigan, New Jersey, New York, and Washington.
We seek to train a diverse community of leaders and encourage applicants from backgrounds which are underrepresented in medicine to apply. High priority will be given to applicants who plan to:
The application deadline is December 1, 2020. Applications will be reviewed on a rolling basis.
For more information about individual fellowship sites and how to apply, please visit our fellowship webpage.
Your gift allows us to train and support health care providers across the United States so they can offer patients compassionate and comprehensive care.
Nov 06
